Description of Procedure or Service
The inherited peripheral neuropathies are a heterogeneous group of diseases that may be inherited in an autosomal dominant, autosomal recessive, or X-linked dominant manner. The inherited peripheral neuropathies can be divided into hereditary motor and sensory neuropathies (such as Charcot-Marie-Tooth disease), hereditary neuropathy with liability to pressure palsies, hereditary sensory and autonomic neuropathies, and other miscellaneous types (e.g., hereditary brachial plexopathy, giant axonal neuropathy). In addition to clinical presentation, nerve conduction studies, and family history, genetic testing can be used to diagnose specific inherited peripheral neuropathies.
When pursuing genetic testing for inherited peripheral neuropathies, genetic counseling is strongly recommended.
Related Policies:
Nerve Fiber Density Testing AHS – M2112
General Genetic Testing, Germline Disorders AHS – M2145
General Genetic Testing, Somatic Disorders AHS – M2146
Prenatal Screening (Genetic) AHS – M2179
Celiac Disease Testing AHS – G2043
***Note: This Medical Policy is complex and technical. For questions concerning the technical language and/or specific clinical indications for its use, please consult your physician.
Policy
BCBSNC will provide coverage for genetic testing for diagnosis of inherited peripheral neuropathies when it is determined the medical criteria or reimbursement guidelines below are met.
Benefits Application
This medical policy relates only to the services or supplies described herein. Please refer to the Member's Benefit Booklet for availability of benefits. Member's benefits may vary according to benefit design; therefore, member benefit language should be reviewed before applying the terms of this medical policy.
When Genetic Testing for Diagnosis of Inherited Peripheral Neuropathies is covered
- For individuals with clinical features of Charcot-Marie-Tooth (CMT) disease, but for whom a definitive diagnosis cannot be made without genetic testing, genetic testing for PMP22 deletions/duplication, GJB1pathogenic or likely pathogenic (P/LP) variants, and/or MFN2 P/LP variants is considered medically necessary.
- For individuals with clinical features of CMT disease who have tested negative for common deleterious variants (PMP22 deletions/duplication; GJB1 or MFN 2P/LP variants), single gene or multi-gene panel testing for CMT disease risk genes is considered medically necessary.
- For asymptomatic individuals who have a close blood relative (see Note 1) with a known deleterious P/LP variant in a CMT gene, genetic testing for the known familial mutation is considered medically necessary.
- For individuals who are clinically suspected of having hereditary neuropathy with liability to pressure palsies (HNPP), but for whom a definitive diagnosis cannot be made without genetic testing, genetic testing for PMP22 deletions and duplications is considered medically necessary.
- For individuals who are clinically suspected of having hereditary motor neuropathy (HMN), but for whom a definitive diagnosis cannot be made without genetic testing, genetic testing for BSCL2 P/LP variants is considered medically necessary.
Note 1: Close blood relatives include first-degree relatives (e.g., parents, siblings, and children), second-degree relatives (e.g., grandparents, aunts, uncles, nieces, nephews, grandchildren, and half-siblings), and third-degree relatives (great-grandparents, great-aunts, great-uncles, great-grandchildren, and first cousins).
Note 2: For two or more gene tests being run on the same platform please refer to AHS-R2162 Laboratory Procedures Medical Policy.
When Genetic Testing for Diagnosis of Inherited Peripheral Neuropathies is not covered
Genetic Testing for Diagnosis of Inherited Peripheral Neuropathies is not covered when the above criteria are not met.
Policy Guidelines
Background
Peripheral neuropathies encompass the set of disorders that primarily lead to peripheral nerve dysfunction. Symptoms typically include weakness of muscles at extremities, spine curvature, and loss of sensation at extremities. Neuropathies may be caused by a variety of different factors, such as metabolic issues (including Fabry disease, Niemann-Pick disease, etc.) or present as a secondary symptom to another condition (such as Tangier disease).
Charcot-Marie-Tooth (CMT) disease, also known as hereditary motor sensory neuropathy, is a group of progressive disorders that affect the peripheral nerves. CMT is caused by a mutation in one of several myelin genes that result in defects in myelin structure, maintenance, or function within peripheral nerves. CMT disease is one of the most common inherited neurological disorders, affecting approximately one in 2,500 people in the United States.
Symptoms
The neuropathy of CMT affects both motor and sensory nerves. Symptoms usually start in childhood and have a gradual progression. The severity of symptoms varies greatly among individuals and even among family members with the disease and gene mutation. Typical symptoms include the following:
- “Weakness or paralysis in the foot and lower leg muscles, making it hard to lift the foot (foot drop)
- A high-stepping walking pattern with frequent tripping or falling
- Balance problems
- Foot deformities, like high arches and curled toes (hammertoes)
- Lower legs with an "inverted champagne bottle" shape due to the loss of muscle bulk
- Trouble feeling heat, cold, and touch
- Possible hand weakness and atrophy, causing difficulty with small, precise movements
- Decreased ability to sense vibrations or know body position (proprioception)
- Curved spine (scoliosis)
- A hip joint out of its normal position (hip displacement)
- A chronic shortening of muscles or tendons around joints (contractures)
- Muscle cramps
- Nerve pain.”
Pain can range from mild to severe, and some people may need to rely on foot or leg braces or other orthopedic devices to maintain mobility. Some people living with CMT experience tremor, and vision and hearing can also be affected. In rare cases, breathing difficulties may occur if the nerves that control the muscles of the diaphragm are affected.
Causes
Charcot-Marie-Tooth is caused by mutations in genes that produce proteins involved in the structure and function of either the peripheral nerve axon or the myelin sheath. Although different proteins are abnormal in different forms of CMT disease, all mutations affect the normal function of the peripheral nerves. There is little correlation between the genotype and phenotype of CMT; it is common to see differing mutations result in various clinical phenotypes all within the same gene.
Pattern of Inheritance
The pattern of inheritance varies with the type of CMT disease. Charcot-Marie-Tooth disease type 1 (CMT1), most cases of Charcot-Marie-Tooth disease type 2 (CMT2), and most intermediate forms are inherited in an autosomal dominant pattern. Charcot-Marie-Tooth disease type 4 (CMT4), a few CMT2 subtypes, and some intermediate forms are inherited in an autosomal recessive pattern. Charcot-Marie-Tooth disease type X (CMTX) is inherited in an X-linked pattern. Some cases of CMT disease result from a new mutation and occur in people with no history of the disorder in their family. In rare cases the gene mutation causing CMT disease is a new mutation which occurs spontaneously in the individual's genetic material and has not been passed down through the family.
CMT1
Charcot-Marie-Tooth disease type 1 (CMT1) is a demyelinating peripheral neuropathy characterized by distal muscle weakness and atrophy, sensory loss, and are usually slowly progressive and often associated with pes cavus foot deformity and bilateral foot drop. The six subtypes of CMT1 shown in Table 1 are clinically indistinguishable and are designated solely on molecular findings.
Table 1: Molecular Genetics of CMT1
Locus Name | Proportion of CMT1 (excluding CMTX) | Gene | Protein Product |
|---|---|---|---|
CMT1A | 70%-80% | PMP22 | Peripheral myelin protein 22 |
CMT1B | 10%-12% | MPZ | Myelin protein P0 |
CMT1C | ~1% | LITAF | Lipopolysaccharide induced tumor necrosis factor-alpha factor |
CMT1D | Unknown | EGR2 | Early growth response protein 2 |
CMT1E | ~1% | PMP22 | Peripheral myelin protein 22 |
CMT1F/2E | Unknown | NEFL | Neurofilament light polypeptide |
Charcot-Marie-Tooth disease type 1A (CMT1A) is an autosomal dominant disease that results from a duplication of the gene on chromosome 17 that carries the instructions for producing the peripheral myelin protein-22 (PMP-22). Overexpression of this gene causes the structure and function of the myelin sheath to be abnormal. A different neuropathy distinct from CMT1A called hereditary neuropathy with predisposition to pressure palsy (HNPP) is caused by a deletion of one of the PMP-22 genes. In this case, abnormally low levels of the PMP-22 gene result in episodic, recurrent demyelinating neuropathy.
Charcot-Marie-Tooth disease type 1B (CMT1B) is an autosomal dominant disease caused by mutations in the gene that carries the instructions for manufacturing the MPZ, which is another critical component of the myelin sheath. Most of these mutations are point mutations. As a result of abnormalities in MPZ, CMT1B produces symptoms similar to those found in CMT1A.
Other less common causes genetic causes of CMT1 result from mutations within LITAF, EGR2, PMP22, and NEFL genes, respectively.
CMT2
Charcot-Marie-Tooth disease type 2 (CMT2) is an axonal (non-demyelinating) peripheral neuropathy characterized by distal muscle weakness and atrophy. Axonal peripheral neuropathy shows extensive clinical overlap with CMT1. In general, individuals with CMT2 tend to be less disabled and have less sensory loss than individuals with CMT1. It is less common than CMT1. CMT2A, the most common axonal form of CMT, is caused by mutations in Mitofusin 2, a protein associated with mitochondrial fusion. Symptoms are similar to those seen in CMT1, but people with CMT2 often have less disability and sensory loss than individuals with CMT1. Additionally, symptoms for CMT2 may have vocal cord or phrenic nerve involvement, causing speech or respiratory problems.
Table 2: Molecular Genetics of CMT2
Locus | Proportion of CMT | Gene / Chromosome Locus | Protein Product |
|---|---|---|---|
CMT2A1 | Unknown | KIF1B | Kinesin-like protein KIF1B |
CMT2A21 | 20% | MFN2 | Mitofusin-2 |
CMT2B | Unknown | RAB7A | Ras-related protein Rab-7 |
CMT2B1 | Unknown | LMNA | Lamin A/C |
CMT2B2 | Unknown | MED25 | Mediator of RNA polymerase II transcription subunit 25 |
CMT2C2 | Unknown | TRPV4 | Transient receptor potential cation channel subfamily V member 4 |
CMT2D3 | 3% | GARS | Glycyl-tRNA synthetase |
CMT2E/1F4 | 4% | NEFL | Neurofilament light polypeptide |
CMT2F | Unknown | HSPB1 | Heat-shock protein beta-1 |
CMT2G | Unknown | 12q12-q13 | Unknown |
CMT2H/2K | 5% | GDAP1 | Ganglioside-induced differentiation-associated protein-1 |
CMT2I/2J | Unknown | MPZ | Myelin protein P0 |
CMT2L | Unknown | HSPB8 | Heat-shock protein beta-8 |
CMT2N | Unknown | AARS | Alanine--tRNA ligase, cytoplasmic |
CMT2O | Unknown | DYNC1H1 | Cytoplasmic dynein 1 heavy chain 1 |
CMT2P | Unknown | LRSAM1 | E3 ubiquitin-protein ligase LRSAM1 |
CMT2S | Unknown | IGHMBP2 | DNA-binding protein SMUBP-2 |
CMT2T | Unknown | DNAJB2 | DnaJ homolog subfamily B member 2 |
CMT2U | Unknown | MARS | Methionine--tRNA ligase, cytoplasmic |
CMT3
Dejerine-Sottas disease (CMT3), is a severe demyelinating neuropathy that begins in infancy. Infants have severe muscle atrophy, weakness, and sensory problems. This rare disorder can be caused by mutations in multiple genes, including PMP22, MPZ, and EGR2, and can be inherited either dominantly or recessively.
CMT4
Charcot-Marie-Tooth disease type 4 (CMT4) comprises several different subtypes of autosomal recessive demyelinating motor and sensory axonal neuropathies. Each neuropathy subtype is caused by a different genetic mutation, may affect a particular ethnic population, and produces distinct physiologic or clinical characteristics. Affected individuals have the typical CMT phenotype of distal muscle weakness and atrophy associated with sensory loss and, frequently, pes cavus foot deformity. Several genes have been identified as causing CMT4, including GDAP1 (CMT4A), MTMR13 (CMT4B1), MTMR2 (CMT4B2), SH3TC2 (CMT4C), NDG1 (CMT4D), EGR2 (CMT4E), PRX (CMT4F), FDG4 (CMT4H), and FIG4 (CMT4J).
Table 3: Molecular Genetics of CMT4
Locus Name | Proportion of CMT4 | Gene | Protein Product |
|---|---|---|---|
CMT4A1 | Unknown | GDAP1 | Ganglioside-induced differentiation-associated protein 1 |
CMT4B1 | MTMR2 | Myotubularin-related protein 2 | |
CMT4B2 | SBF2 | Myotubularin-related protein 13 | |
CMT4C2 | SH3TC2 | SH3 domain and tetratricopeptide repeats-containing protein 2 | |
CMT4D | NDRG1 | Protein NDRG1 | |
CMT4E | EGR2 | Early growth response protein 2 | |
CMT4F | PRX | Periaxin | |
CMT4H3 | FGD4 | FYVE, RhoGEF and PH domain-containing protein 4 | |
CMT4J4 | FIG4 | Phosphatidylinositol 3, 5 biphosphate |
CMTX
Charcot-Marie-Tooth disease type X (CMTX) is caused by a point mutation in the connexin-32 gene on the X chromosome. The connexin-32 protein is expressed in Schwann cells, which wrap around nerve axons and make up a single segment of the myelin sheath. CMTX type 1 is characterized by a moderate to severe motor and sensory neuropathy. Hearing loss and central nervous system symptoms may also occur in certain affected families.
Table 4: Molecular Genetics of CMTX
| Disease Name | Proportion of X-Linked CMT | Gene / Chromosome Locus | Protein Product |
|---|---|---|---|
| CMTX11 | 90% | GJB1 | Gap junction beta-1 protein (connexin 32) |
| CMTX22 | Unknown | Xp22.2 | |
| CMTX31 | Not applicable | ||
| CMTX41 | AIFM1 | Apoptosis-inducing factor 1 | |
| CMTX52 | PRPS1 | Ribose-phosphate pyrophosphokinase 1 | |
| CMTX61 | PDK3 | Pyruvate dehydrogenase kinase isoform 3 |
Hereditary Brachial Plexopathy (Hereditary Neuralgic Amyotrophy)
This condition is primarily characterized by painful injuries to the brachial plexus nerves as well as episodic weakness of the shoulder and arm. Other symptoms such as winging of the scapula, short stature, neck folds, small face, and hypotelorism may be present. Nerve conduction velocity is typically normal, and the histopathology of this condition is non-specific. The septin 9 gene (SEPT9) on chromosome 17 has been associated with this condition.
Giant Axonal Neuropathy
This condition is characterized by disorganization of cytoskeletal intermediate filaments stemming from a mutated form of gigaxonin. Patients with this disorder often have a signature physical appearance; red and kinked hair, high foreheads, long eyelashes, and pale complexions are all hallmarks of this condition. The central nervous system may be affected as well with cerebellar dysfunction, spasticity, and potentially intellectual disability as possible symptoms. Nerve biopsy may show axonal loss or another axonal dysfunction. This diagnosis is confirmed by testing of the GAN gene.
Hereditary Sensory and Autonomic Neuropathies (HSANs)
This subsection of disorders primarily encompasses non-motor neuropathies and are characterized by major loss of myelinated and unmyelinated fibers. These conditions are not as common as hereditary motor neuropathies and primarily present with sensory dysfunction, although motor functions may be affected. There are multiple main types of HSAN, each caused by different genes. Genes are associated as shown below:
Disorder | Gene | Clinical features |
|---|---|---|
| HSAN1 | SPTLC1 | Most are autosomal dominant |
SPTLC2 | Onset often in early adulthood but variable | |
ATL1 | Distal sensory loss, foot ulcers | |
DNMT1 | Preservation of facial sensation | |
ATL3 | Variable muscle wasting and weakness | |
| Variable neural deafness and dementia | |
| HSAN2 | WNK1/HSN2 | Autosomal recessive |
FAM134B | Loss of pain, temperature, and tactile sensation | |
KIF1A | Recurrent infection and fractures of the digits | |
SCN9A |
| |
| HSAN3 (familial dysautonomia) | IKBKAP | Autosomal recessive |
Progressive sensorimotor neuropathy | ||
Sympathetic autonomic dysfunction | ||
Smooth tongue without fungiform papillae | ||
| HSAN4 (congenital insensitivity to pain with anhidrosis) | NTRK1 | Autosomal recessive |
Profound loss of pain sensitivity | ||
Defects in thermoregulation | ||
Anhydrosis | ||
Mild to moderate mental retardation | ||
Microcephaly | ||
Fungiform papillae are present | ||
| HSAN5 | NGFB | Autosomal recessive |
Loss of pain and temperature sensation | ||
Normal muscle strength | ||
Normal reflexes | ||
Normal nerve conduction | ||
| HSAN6 | DST | Autosomal recessive; Ashkenazi Jewish |
Autonomic dysfunction | ||
Absent fungiform papillae | ||
Death by age 2 years | ||
| HSAN7 | SCN11A | Autosomal dominant |
Congenital insensitivity to pain | ||
Self-mutilation, slow wound healing, painless bone fractures | ||
Gastrointestinal dysfunction | ||
Hyperhidrosis | ||
| HSAN8 | PRDM12 | Autosomal recessive |
Self-mutilation, insensitivity to pain | ||
Soft tissue injuries | ||
Corneal scarring | ||
Hypohidrosis | ||
| HSAN and dementia | PRNP | Autosomal dominant |
Dementia | ||
Autonomic dysfunction | ||
Sensory loss | ||
| Hereditary sensory neuropathy with spastic paraplegia | CCT5 | Autosomal recessive |
Spastic paraplegia | ||
Ulcerations of hands and feet | ||
Insensitivity to pain | SCN9A | Autosomal recessive: |
Paroxysmal extreme pain disorder | Insensitivity to pain | |
Primary erythermalgia | Autosomal dominant: | |
Small fiber neuropathy
| Paroxysmal extreme pain disorder | |
Primary erythermalgia | ||
Small fiber neuropathy |
AD: autosomal dominant; AR: autosomal recessive; HSAN: hereditary sensory and autonomic neuropathy
Other unclassified HSANs exist, such as spastic paraplegia with ulcerations of the hands and feet (associated with CCT5) and sensory neuropathy with ichthyosis and anterior chamber syndrome.
Genetic Testing
Charcot-Marie-Tooth disease is usually diagnosed by an extensive history and physical examination. The clinical diagnosis is then confirmed by electrodiagnostic tests like electromyography and nerve conduction velocity tests, and sometimes by nerve biopsy. Genetic testing is available for most types of CMT, and results are usually enough to confirm a diagnosis. Genetic testing can simplify the diagnosis of CMT by avoiding invasive procedures, such as nerve biopsy. In addition, early diagnosis can facilitate early interventions, including physical therapy. However, most therapies are only supportive (occupational, physical) and generally do not rely on the results of specific genetic testing. A positive genetic test can confirm diagnosis in most people with CMT. But a negative result does not exclude the disease, as an unidentified gene may be missed by DNA sampling.
Genetic testing for CMT is complicated by the extensive underlying genetic heterogeneity. The CMT spectrum of disorders can be inherited in an autosomal dominant, autosomal recessive, or X-linked manner. The most commonly identified CMT subtypes are CMT1A (PMP22 duplication), CMTX1 (GJB1 mutation), hereditary neuropathy with liability to pressure palsies (PMP22 deletion), CMT1B (MPZ mutation), and CMT2A (MFN2 mutation). Together, these five subtypes account for 92 percent of genetically defined CMT cases. All other CMT subtypes and associated mutations each account for <1 percent of genetically defined CMT. Genetic screening for relatives of a patient diagnosed with CMT is an option, but risk assessment depends on several factors, including accuracy of the diagnosis, determination of the mode of inheritance for the individual family, and results of molecular genetic testing.
Proprietary Testing
Numerous genetic panels are available for the assessment of peripheral neuropathies, such as GeneDx’s panel (64 genes) and Invitae’s panel (83 genes). Other pane’s include ones by Athena Diagnostics (23 genes),MNG Laboratories (139 genes), Prevention Genetics (44 genes), and Variantyx’s Genomic Unity panel (25 genes).
Clinical Utility and Validity
DiVincenzo, et al. (2014) performed an analysis of the genetic landscape of CMT. A total of 14 genes associated with CMT (PMP22, GJB1, MPZ, MFN2, SH3TC2, GDAP1, NEFL, LITAF, GARS, HSPB1, FIG4, EGR2, PRX, and RAB7A) were evaluated out of 3312 individuals. Deletions and duplications in the PMP22 gene consisted of about 78% of positive findings, followed by mutations in the GJB1 (6.7%), MPZ (5.3%), and MFN2 (4.3%) genes. A total of 71% of the pathogenic mutations found were missense mutations. Overall, 95% of the positive results involved one of four genes (PMP22, GJB1, MPZ, MFN2). The authors conclude that these four genes should be screened first before proceeding with further genetic testing.
Pareyson, et al. (2017) reviewed the current literature on CMT diagnosis stating that data justifies a stepwise algorithm considering a variety of factors, such as phenotype, nerve conduction velocities, and ethnicity. The authors note that next-generation sequencing (NGS) is steadily replacing older methods of sequencing in this algorithm. The authors propose evaluating the first few common genes (PMP22, MPZ, et al) and then considering larger sequencing methods such as NGS. However, due to the growing number of genes associated with CMT, these larger sequencing methods may be considered first-line. Finally, the authors state that due to the growing number of associated genes, newer classifications need to be discussed and validated further.
Rudnik-Schöneborn, et al. (2016) evaluated the clinical features and genetic results of 1206 CMT patients and 124 affected relatives. Genetic detection rates were 56% in demyelinating CMT and 17% in axonal CMT. “Three genetic defects (PMP22 duplication/deletion, GJB1/Cx32 or MPZ/P0 mutation) were responsible for 89.3% of demyelinating CMT index patients in whom a genetic diagnosis was achieved, and the diagnostic yield of the three main genetic defects in axonal CMT (GJB1/Cx32, MFN2, MPZ/P0 mutations) was 84.2%.” The authors concluded that “diagnostic algorithms are still useful for cost-efficient mutation detection and for the interpretation of large-scale genetic data made available by next generation sequencing strategies.”
Vaeth, et al. (2019) evaluated the effect of implementing a targeted NGS approach for identifying CMT. The authors stated that from 1992-2012, a total of 1442 CMT analyses were performed (through Sanger sequencing and other quantitative analyses) and a pathogenic variant was discovered in 21.6% of these cases. From this cohort, 195 samples that did not reach a definitive diagnosis were sequenced by a custom 63-gene panel. The authors identified a 5.6% increase in diagnostic yield using this targeted NGS approach.
Cortese, et al. (2020) investigated the effectiveness of NGS panels in CMT. A total of 220 patients were enrolled in the study and a targeted CMT NGS panel was performed. After NGS sequencing, a molecular diagnosis based on a pathogenic variant was found in 30% of the cases and variants of unknown significance were found in 33% of the cases. A total of 39% of the cases held mutations in GJB1, MFN2, and MPZ while the others held mutations in SH3TC2, GDAP1, IGHMBP2, LRSAM1, FDG4, and GARS. Copy number changes were detected in PMP22, MPZ, MFN2, SH3TC2, and FDG4. The authors conclude that "NGS panels are effective tools in the diagnosis of CMT, leading to genetic confirmation in one-third of cases negative for PMP22 duplication/deletion, thus highlighting how rarer and previously undiagnosed subtypes represent a relevant part of the genetic landscape of CMT.”
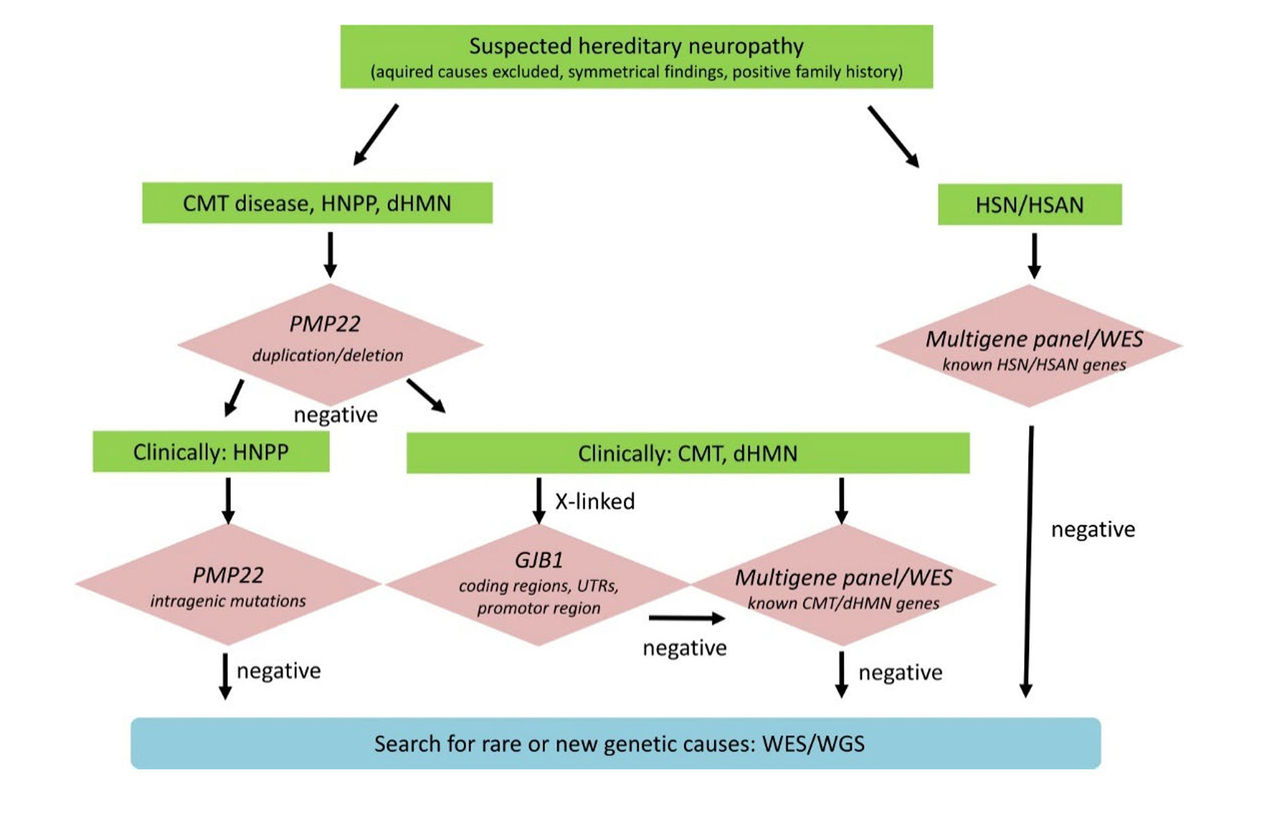
Rudnik-Schöneborn, et al. (2020) suggested a diagnostic algorithm for genetic testing of suspected hereditary neuropathy. Advanced genetic sequencing allows for comprehensive evaluation of the pathogenic relevance of identified variants. As shown in the chart above, “If PMP22 copy number analysis is negative, then clinical distinction of HNPP and CMT/dHMN will sort out patients for PMP22 mutation analysis only and those for broader multigene testing. If a pedigree is compatible with X-linked inheritance, it is recommended to analyze coding and non-coding regions of GBJ1. Patients who are tested negative for known neuropathy genes may be included in further whole exome or genome sequencing (WES/WGS) to detect mutations in rare and new genes.”
Yalcintepe, et al. (2021) studied the importance of multiple gene analysis for diagnosis of Charcot Marie Tooth Disease. Fifty-five patients with suspected CMT phenotype were examined using a customized multi-gene panel which was compared to the Multiplex Ligand Probe Amplification method. The custom panel identified 13 cases (7.15%) with a pathogenic/likely pathogenic variant. “The affected genes were MARS1, NDRG1, GJB1, GDAP1, MFN2, PRX, SH3TC2, and FGD4. Six cases (10.9%) had pathogenic variants in GJB1 and FGD4 genes, variants of unknown significance (VUS) in HSPB3, CHRNA1, ARHGEF10, and KIF5A genes. A total of 21 cases (11.55%) had VUS with the genes HSPB3, KIF1B, SCN11A, CHRNA1, HSPB1, FIG4, ARHGEF10, DHTKD1, SBF1, EGR2, SBF2, IGHMBP2, KIF5A, and DNAJB2.” The authors concluded that the NGS customized panel was beneficial, time-saving, and cost-effective in the diagnosis of CMT.
Guidelines and Recommendations
American Academy of Neurology (AAN), the American Academy of Neuromuscular and Electrodiagnostic Medicine (AANEM), and the American Academy of Physical Medicine and Rehabilitation (AAPM&R)
The Polyneuropathy Task Force that included 19 physicians with representatives from the AAN, AANEM, and AAPM&R concluded that “genetic testing is established as useful for the accurate diagnosis and classification of hereditary polyneuropathies (Class I).”
The Task Force stated that “for patients with a cryptogenic polyneuropathy who exhibit a classic hereditary neuropathy phenotype, routine genetic screening may be useful for CMT1A duplication/deletion and Cx32 mutations in the appropriate phenotype (Class III). Further genetic testing may be considered guided by the clinical question.” The Task Force recommended that “genetic testing should be conducted for the accurate diagnosis and classification of hereditary neuropathies (Level A).” The Task Force further recommended that “Genetic testing may be considered in patients with a cryptogenic polyneuropathy and classic hereditary neuropathy phenotype (Level C). Initial genetic testing should be guided by the clinical phenotype, inheritance pattern, and electrodiagnostic features and should focus on the most common abnormalities which are CMT1A [PMP22] duplication/HNPP deletion, Cx32 (GJB1), and MFN2 mutation screening. There is insufficient evidence to support or refute the usefulness of routine genetic testing in cryptogenic polyneuropathy patients without a classic hereditary phenotype (Level U).”
These guidelines were reaffirmed on February 8, 2025.
European Federation of Neurological Societies (EFNS)
The EFNS released recommendations on genetic testing for various types of peripheral neuropathies. Regarding CMT, they noted that “given the rarity of AR CMT in the European population routine diagnostic screening of the many known genes is currently not feasible” but acknowledged that “currently, molecular genetic testing can be offered for several of the more prevalent CMT genes.” EFNS stated that PMP22 duplication should be tested first in patients presenting with CMT1, followed by sequencing of GJB1, MPZ, and PMP22. If a patient presents with CMT2, MFN2 should be screened first, followed by MPZ. If a patient presents with intermediate CMT, GJB1 and MPZ should be screened. EFNS notes that in patients with hereditary neuropathy with liability to pressure palsies will be investigated for a PMP22 deletion at the same time as a screening for a PMP22 duplication.
However, routine diagnostic screenings for hereditary motor neuropathy (HMN) and hereditary sensory-autonomic neuropathy (HSAN) are not feasible due to low mutation frequencies. If screening is performed for these conditions, EFNS recommends BSCL2 as the first candidate for screening in HMN. NTRK1 may also be screened for in congenital insensitivity to pain with anhidrosis patients (CIPA, a sub-phenotype of HSAN) and RAB7 may be screened in CMT2B patients. Finally, SEPT9 may be screened in the context of hereditary neuralgic amyotrophy.
State and Federal Regulations, as applicable
Many labs have developed specific tests that they must validate and perform in house. These laboratory-developed tests (LDTs) are regulated by the Centers for Medicare and Medicaid (CMS) as high-complexity tests under the Clinical Laboratory Improvement Amendments of 1988 (CLIA ’88). LDTs are not approved or cleared by the U. S. Food and Drug Administration; however, FDA clearance or approval is not currently required for clinical use.
Billing/Coding/Physician Documentation Information
This policy may apply to the following codes. Inclusion of a code in this section does not guarantee that it will be reimbursed. For further information on reimbursement guidelines, please see Administrative Policies on the Blue Cross Blue Shield of North Carolina web site at www.bcbsnc.com. They are listed in the Category Search on the Medical Policy search page.
Applicable service codes: 81324, 81325, 81326, 81403, 81404, 81405, 81406, 81448
BCBSNC may request medical records for determination of medical necessity. When medical records are requested, letters of support and/or explanation are often useful but are not sufficient documentation unless all specific information needed to make a medical necessity determination is included.
Scientific Background and Reference Sources
Kang P. Overview of hereditary neuropathies. Updated April 15, 2024. https://www.uptodate.com/contents/overview-of-hereditary-neuropathies
UpToDate. Patient education: Charcot-Marie-Tooth disease (The Basics). https://www.uptodate.com/contents/charcot-marie-tooth-disease-the-basicas
Kang P. Charcot-Marie-Tooth disease: Genetics, clinical features, and diagnosis. Updated March 3, 2025. https://www.uptodate.com/contents/charcot-marie-tooth-disease-genetics-clinical-features-and-diagnosis
Bird T. Charcot-Marie-Tooth (CMT) Hereditary Neuropathy Overview. 2025. https://www.ncbi.nlm.nih.gov/books/NBK1358/
NINDS. Charcot-Marie-Tooth Disease. https://www.ninds.nih.gov/health-information/disorders/charcot-marie-tooth-disease
Saporta AS, Sottile SL, Miller LJ, Feely SM, Siskind CE, Shy ME. Charcot-Marie-Tooth disease subtypes and genetic testing strategies. Annals of neurology. Jan 2011;69(1):22-33. doi:10.1002/ana.22166
Züchner S. Charcot-Marie-Tooth Neuropathy Type 2A. 2025. https://www.ncbi.nlm.nih.gov/books/NBK1511/
Schindler A. TRPV4-Associated Disorders. 2020. https://www.ncbi.nlm.nih.gov/books/NBK201366/
Antonellis A, Goldfarb LG, Sivakumar K. GARS-Associated Axonal Neuropathy. 2021. https://www.ncbi.nlm.nih.gov/books/NBK1242/
DeJonghe P, Jordanova AK. Charcot-Marie-Tooth Neuropathy Type 2E/1F. 2011. https://www.ncbi.nlm.nih.gov/books/NBK1187/
Bird T. GDAP1-Related Hereditary Motor and Sensory Neuropathy. 2017. https://www.ncbi.nlm.nih.gov/books/NBK1539/
Hamid Azzedine EL, and Mustafa A Salih. Charcot-Marie-Tooth Neuropathy Type 4C. 2021. https://www.ncbi.nlm.nih.gov/books/NBK1340/
Delague V. Charcot-Marie-Tooth Neuropathy Type 4H. 2013. https://www.ncbi.nlm.nih.gov/books/NBK153601/
Li J. Charcot-Marie-Tooth Neuropathy Type 4J. 2013. https://www.ncbi.nlm.nih.gov/books/NBK169431/
Abrams C. GJB1 Disorders: Charcot Marie Tooth Neuropathy (CMT1X) and Central Nervous System Phenotypes. 2024. https://www.ncbi.nlm.nih.gov/books/NBK1374/
Kim J-W, Kim H-J. Charcot-Marie-Tooth Neuropathy X Type 5. 2013. https://www.ncbi.nlm.nih.gov/books/NBK1876/
Bromberg MB. Brachial plexus syndromes. Updated April 4, 2024. https://www.uptodate.com/contents/brachial-plexus-syndromes
Eichler F. Hereditary sensory and autonomic neuropathies. Updated February 17, 2025. https://www.uptodate.com/contents/hereditary-sensory-and-autonomic-neuropathies
Kang P. Charcot-Marie-Tooth disease: Management and prognosis. Updated August 14, 2024. https://www.uptodate.com/contents/charcot-marie-tooth-disease-management-and-prognosis
Charcot-Marie-Tooth News. Genetic Tests and Genetic Counseling. https://charcot-marie-toothnews.com/genetic-tests/
CMTA. Genetic Testing. https://www.cmtausa.org/living-with-cmt/find-help/genetic-testing/
GeneDx. Hereditary Neuropathy Panel. https://www.genedx.com/tests/detail/hereditary-neuropathy-panel-800
Invitae. Invitae Comprehensive Neuropathies Panel. https://www.invitae.com/us/providers/test-catalog/test-03200
Athena Diagnostics. CMT Advanced Evaluation - Comprehensive. https://www.athenadiagnostics.com/view-full-catalog
MNG Laboratories. Charcot-Marie-Tooth Disease, Axonal (NGS Panel and Copy Number Analysis + mtDNA). https://mnglabs.labcorp.com/testing
Prevention Genetics. Charcot-Marie-Tooth (CMT) - Comprehensive Panel. https://www.preventiongenetics.com/testInfo?val=Charcot-Marie-Tooth+%28CMT%29+-+Comprehensive+Panel
DiVincenzo C, Elzinga CD, Medeiros AC, et al. The allelic spectrum of Charcot-Marie-Tooth disease in over 17,000 individuals with neuropathy. Molecular genetics & genomic medicine. Nov 2014;2(6):522-9. doi:10.1002/mgg3.106
Pareyson D, Saveri P, Pisciotta C. New developments in Charcot-Marie-Tooth neuropathy and related diseases. Current opinion in neurology. Oct 2017;30(5):471-480. doi:10.1097/wco.0000000000000474
Rudnik-Schoneborn S, Tolle D, Senderek J, et al. Diagnostic algorithms in Charcot-Marie-Tooth neuropathies: experiences from a German genetic laboratory on the basis of 1206 index patients. Clinical genetics. Jan 2016;89(1):34-43. doi:10.1111/cge.12594
Vaeth S, Christensen R, Duno M, et al. Genetic analysis of Charcot-Marie-Tooth disease in Denmark and the implementation of a next generation sequencing platform. Eur J Med Genet. Jan 2019;62(1):1-8. doi:10.1016/j.ejmg.2018.04.003
Cortese A, Wilcox JE, Polke JM, et al. Targeted next-generation sequencing panels in the diagnosis of Charcot-Marie-Tooth disease. Neurology. 2020;94(1):e51-e61. doi:10.1212/WNL.0000000000008672
Rudnik-Schöneborn S, Auer-Grumbach M, Senderek J. Charcot-Marie-Tooth disease and hereditary motor neuropathies – Update 2020. Medizinische Genetik. 2020;32(3):207-219. doi:10.1515/medgen-2020-2038
Yalcintepe S, Gurkan H, Dogan IG, et al. The Importance of Multiple Gene Analysis for Diagnosis and Differential Diagnosis in Charcot Marie Tooth Disease. Turk Neurosurg. 2021;31(6):888-895. doi:10.5137/1019-5149.jtn.33661-21.3
England JD, Gronseth GS, Franklin G, et al. Practice Parameter: Evaluation of distal symmetric polyneuropathy: Role of laboratory and genetic testing (an evidence-based review). Neurology. 2009;72(2):185. doi:10.1212/01.wnl.0000336370.51010.a1
Burgunder JM, Schols L, Baets J, et al. EFNS guidelines for the molecular diagnosis of neurogenetic disorders: motoneuron, peripheral nerve and muscle disorders. European journal of neurology. Feb 2011;18(2):207-17. doi:10.1111/j.1468-1331.2010.03069.x
Specialty Matched Consultant Advisory Panel review 7/2019
Medical Director review 7/2019
Specialty Matched Consultant Advisory Panel review 7/2020
Medical Director review 7/2020
Specialty Matched Consultant Advisory Panel review 7/2021
Medical Director review 7/2021
Medical Director review 7/2022
Medical Director review 7/2023
Medical Director review 7/2024
Medical Director review 7/2025
Policy Implementation/Update Information
1/1/2019 BCBSNC will provide coverage for genetic testing for diagnosis of inherited peripheral neuropathies when it is determined to be medically necessary because criteria and guidelines are met. Medical Director review 1/1/2019. Policy noticed 1/1/2019 for effective date 4/1/2019. (jd)
8/13/2019 Specialty Matched Consultant Advisory Panel review 7/2019. Medical Director review 7/2019 (jd)
9/10/2019 Reviewed by Avalon 2nd Quarter 2019 CAB. Related Policies added to Description section. When Covered section revised as follows: Item 1, added “or other inherited peripheral neuropathies”; item 2, added “If results indicate a demyelinating neuropathy, then first test for the most commonly identified CMT subtype, CMT1A (PMP22 duplication.”, and removed items a. and b. related to specific values for velocity testing in nerve conduction and specific cascade testing; added item 6 for Genetic testing for Hereditary Motor Neuropathy (HMN) (BSCL2 gene), and added “Note” which refers to policy, General Genetic Testing, Germline Disorders AHS – M2145 for all other uncommon hereditary peripheral neuropathy gene testing. Removed the following statement from the When Not Covered section: “Genetic testing for all other inherited peripheral neuropathies is considered investigational”. Code table removed from the Billing/Coding section and reimbursement statement added. Policy guidelines and references updated. Medical Director review. (jd)
10/29/19 Wording in the Policy, When Covered, and/or Not Covered section(s) changed from Medical Necessity to Reimbursement language, where needed. (hb)
7/28/20 Reviewed by Avalon 2nd Quarter 2020 CAB. Policy guidelines and references updated. Specialty Matched Consultant Advisory Panel review 7/2020. Medical Director review 7/2020. (jd)
8/24/21 Reviewed by Avalon 2nd Quarter 2021 CAB. The following statement was added to the When Not Covered section: “Genetic testing for CMT in asymptomatic individuals is considered investigational.” Background, policy guidelines, and references updated. Specialty Matched Consultant Advisory Panel review 7/2021. Medical Director review 7/2021. (jd)
9/13/22 Reviewed by Avalon 2nd Quarter 2022 CAB. Updated background, guidelines, and references. Billing/Coding section updated. Coverage criteria edited for clarity. No change to policy statement. Medical Director review 7/2022. (tm)
8/15/23 Reviewed by Avalon 2nd Quarter 2023 CAB. Updated Description, Policy Guidelines, and References. The following updates were made to the When Covered section: Former coverage criteria 2 split and expanded into new coverage criteria 1 and 2 for full clarity regarding which genes should be ordered first based on prevalence of cause. Recommendation for genetic counseling removed as coverage criteria and moved into the Policy Description. Removed former coverage criteria 4 (prenatal screening addressed in M2179) and coverage criteria 5 regarding peripheral nerve biopsy. previous Note 1 removed and replaced with new Note 1. Medical Director review 7/2023. (tm)
9/4/24 Reviewed by Avalon 2nd Quarter 2024 CAB. Updated Description, Policy Guidelines, and References. Codes 90640 and S0265 removed from Billing/Coding section, Note 2 under When Covered section updated to reflect changes to definition of a genetic panel within R2162. Now reads: “Note 2: For 2 or more gene tests being run on the same platform, please refer to Laboratory Procedures Medical Policy AHS - R2162.” Medical Director review 7/2024. (tm)
10/15/25 Reviewed by Avalon 3rd Quarter 2025 CAB. Updated Policy Guidelines and References. The following edits were made to the When Covered section for clarity: replaced mutation with “pathogenic or likely pathogenic (P/LP) variants” in coverage criteria 1, 2, 4, 5, Note 1 edited to write out “first”, “second”, and “third” for consistency, Note 2 edited to change “2” to “two”. No change to policy statement. Medical Director review 7/2025. (tm)
Blue Cross and Blue Shield of North Carolina does not discriminate on the basis of race, color, national origin, sex, age or disability in its health programs and activities. Learn more about our non-discrimination policy and no-cost services available to you.
Information in other languages: Español 中文 Tiếng Việt 한국어 Français العَرَبِيَّة Hmoob ру́сский Tagalog ગુજરાતી ភាសាខ្មែរ Deutsch हिन्दी ລາວ 日本語
© 2026 Blue Cross and Blue Shield of North Carolina. ®, SM Marks of the Blue Cross and Blue Shield Association, an association of independent Blue Cross and Blue Shield plans. All other marks and names are property of their respective owners. Blue Cross and Blue Shield of North Carolina is an independent licensee of the Blue Cross and Blue Shield Association.