In a case series study of multisystem inflammatory syndrome in adults (MIS-A) associated with SARS-CoV-2 infection, 16 patients ranging from 21 to 50 years old were enrolled and tested with PCR assay. Ten out of 16 patients had positive SARS-CoV-2 PCR test results at the time of admission. Two patients had positive SARS-CoV-2 PCR test results 14 and 37 days before admission and negative PCR results at the time of admission. Three patients had positive SARS-CoV-2 PCR test results 25–41 days before admission and continued positive PCR test results at the time of admission. “Given the high proportion of MIS-C patients with negative PCR testing, clinical guidelines recommend the use of both antibody and viral testing to assist with diagnosis” (Morris et al., 2020).
Li et al. (2021) conducted a cross-sectional analysis on 30 patients with COVID-19 diagnoses to compare the sensitivity of SARS-CoV-2 testing in anterior nasal vestibular swabs versus oropharyngeal swabs. After specimen collection, RT-PCR assays were used to test them for SARS-CoV-2. They found that 56.7% of the patients tested positive using oropharyngeal specimen, whereas 66.7% of patients tested positive with the nasal swab specimens. Ultimately, there is “adequate sensitivity” to use the less invasive anterior nasal vestibular swabs to detect COVID-19 infection confirmed by RT-PCR (Li et al., 2021).
Yau et al. (2021) evaluated the clinical utility of a rapid “on-demand” PCR-based testing service in an acute hospital setting. To increase hospital efficiency starting from July 2020, the researchers focused on moving patients quickly to isolation rooms and minimize potential risk of transmission in crowded areas. From their study, it was found that the “daily/monthly PCR positive test numbers approximately followed the local and national UK trend in COVID-19 case numbers, with the daily case numbers being reflective of the Nov and Dec 2020 surges.” It ultimately helped to reduce “unnecessary ‘length-of-stay’ in a busy acute respiratory ward.” Patients were able to be rapidly separated based on COVID-19 positive diagnosis and the system in place reduced exposure and nosocomial transmission (Yau et al., 2021).
Dighe et al. (2022) studied a lateral flow strip-based RNA extraction and amplification-free nucleic acid test (NAT) for rapid diagnosis of COVID-19 at point of care which takes no longer than 30 minutes. This test uses highly specific 6-carboxyfluorescein (6-FAM) and biotin labeled antisense oligonucleotides (ASOs) as probes those are designed to target the N-gene sequence of COVID-19. This study evaluated 60 samples using the lateral flow assay and results were compared with the FDA approved TaqPath RT-PCR kit. According to the results, the assay obtained almost 99.99% accuracy and specificity. The authors conclude that this new LFA method could be "expanded beyond COVID-19 detection, simply by altering its targeting antisense oligonucleotides, to become a global health technology that contributes to providing low-cost diagnostics" (Dighe et al., 2022).
Mawhorter et al. (2022) investigated the impact and cost of a routine pre-operative COVID-19 PCR testing algorithm for asymptomatic patients before elective surgery at a rural academic institution per recommendations by the American College of Surgeons. From 7579 pre-procedural tests that were completed since May 2020 using the protocol, the study yielded 31 (0.41%) positive results in asymptomatic patients. With these positive results, there were impacts on both the cost and delay of the procedure. The results showed that “20 procedures (62.5%) were delayed an average of 49 days, 8 were not performed, and 3 proceeded without delay,” with a prolonged delay for the three urological procedures of 59 days. They also identified that the number needed to test for one positive result was 244, with $11,573 as cost for each positive result. This analysis found that the hospital was able to be more cost-effective (each test was $34-54) with a standardized testing algorithm prior to procedure performance (Mawhorter et al., 2022).
Host Antibody Testing
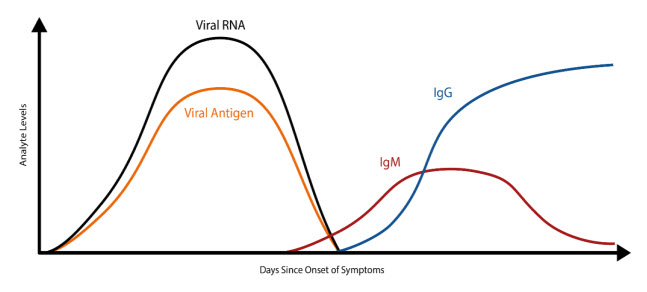
The COVID-19 illness begins with an initial infection by SARS-CoV-2. Viral invasion stimulates the host immune response to produce immunoglobulins, such as IgM, IgA, and IgG, that can target the invading virus. However, there is a delay between the time of initial infection and the production of immunoglobulins (Figure 1) (The Native Antigen Company, 2020). Typically, several days after the initial onset of symptoms, the first IgM immunoglobulins are produced to combat the viral infection. IgA (not shown in Figure 1), immunoglobulins secreted to protect predominantly the mucosal linings of the gastrointestinal, respiratory, and genitourinary tracts (Woof & Kerr, 2006), typically have a half-life of four to six days (Morell et al., 1973). Finally, IgG, the long-term immunoglobulins found within body fluids that fight bacterial and viral infections, are produced and IgM production wanes. Some limited studies have indicated that some individuals may initially produce IgM and IgG antibodies concurrently, but additional research is needed (Padoan et al., 2020).
Serological host antibody tests can detect the presence of IgM and IgG antibodies that an individual has developed in response to an infection—in this case, a SARS-CoV-2 viral infection. The test may report total antibodies present, meaning either it does not distinguish between IgG and IgM or that it is reporting the sum of IgG and IgM. This is sometimes referred to as “total antibody testing.” On the other hand, the test may be specific for one antibody, such as IgG or IgM, or the test may claim to accurately distinguish between the antibodies.
Another type of antibody testing is “neutralizing” antibody detection, as opposed to “binding” antibody detection described above. This process involves incubating serum with a live version of the virus. The analytes of interest are the antibodies that have the capability to prevent infection by the virus (i.e. neutralization). Identification of these antibodies may contain useful clinical information and are often reported in an aggregate titer, as opposed to specifying each individual antibody (Espejo et al., 2020).
Clinical Utility and Validity of Host Antibody Testing
Antibody testing has many potential uses. Ideally, the use of an accurate, reliable antibody test could possibly show whether someone has previously been exposed to the virus. This could indicate possible immunity in an individual. Please note that the antibody test is not used as a diagnostic test, meaning it should not be used to diagnose an acute infection. Within the FDA policy for diagnostic testing for COVID-19, issued on November 15, 2021 they state, “Results from antibody testing should not be used to diagnose or exclude SARS-CoV-2 infection” (FDA, 2023b).
Since SARS-CoV-2 is a new, emerging virus, it is not known for certain how long it takes for the seroconversion to occur or when antibodies start to appear in the blood at high enough concentrations for accurate testing results. A recent study published in Clinical Infectious Diseases reports an average of seroconversion time for IgM and IgG at 12 and 14 days, respectively (Zhao et al., 2020). A small study (n=34 patients) reports the presence of IgG for at least seven weeks (the duration of the study) (Xiao et al., 2020). Another study, however, reports that IgM testing has similar, if not better positive detection rate than PCR 5.5 days after initial onset of symptoms; however, the total window of antibody detection for IgM was only five days long (Guo et al., 2020) (See Figure 1). If the patient was not tested during the detection window, then the individual would not necessarily have a “positive” result for IgM. The authors also report the detection of IgA antibodies (median onset at five days after initial symptoms [IQR three to six days]), and 92.7% of total samples report a positive result for IgA. This same study also reports that IgG detection occurs, on average, fourteen days after initial onset of symptoms (Guo et al., 2020). Another study reports that IgA-based ELISA testing has higher sensitivity than IgG-based ELISA testing, but the IgG-based ELISA testing has higher specificity. The authors recommend IgG-based testing over the IgA-based testing in immunosurveillance studies since IgG has a longer biological half-life (Okba et al., 2020). At least one published study to date has reported that as many as 6.9% of individuals who previously had tested positive with RT-PCR results did not show the presence of antibodies for the length of the study (at least 40 days after the initial onset of symptoms) (Zhao et al., 2020).
Ideally, any rapid diagnostic test for the outpatient setting must be accurate and reliable. Current research indicates that the diagnostic window for IgA and IgM is very limited. Some data indicate that host antibody testing can also yield inaccuracies. Also, for IgG testing, the significance of positive results is questionable at the current time. A positive result could indicate a previous infection, assuming the test did not cross-react with any other IgG the host produced in response to one of the four coronaviruses known to cause the common cold in humans, for example. It is not currently known, however, if the presence of IgG antibodies indicates immunity (or degree thereof) of the host against SARS-CoV-2. The duration of any conferred immunity, or the level of IgG antibodies required to effectively acquire such immunity, are also unknown. Additional research is needed and encouraged.
Lisboa Bastos et al. (2020) performed a meta-analysis to investigate the diagnostic accuracy of serological testing for COVID-19. The authors aimed to identify studies where serological testing was compared to the “reference standard of viral culture or reverse transcriptase polymerase chain reaction.” The authors identified a total of 40 studies for inclusion in the study. The pooled sensitivity of enzyme linked immunosorbent assays (ELISAs) measuring IgG or IgM to be 84.3% (with a 95% confidence interval [CI] of 75.6%-90.9%). For lateral flow immunoassays (LFIAs), the pooled sensitivity was found to be 66% (95% CI: 49.3%-79.3%), and for chemiluminescent immunoassays (CLIAs), the pooled sensitivity was found to be 97.8% (95% CI: 46.2%-100%). Pooled specificities ranged from 96.6%-99.7%. Sensitivity was also found to be higher at least three weeks from symptom onset (69.9% to 98.9%) compared to within the first week (13.4% to 50.3%) Of the samples used to calculate specificity, 83% were “from populations tested before the epidemic or not suspected of having COVID-19.” The authors performed 49 bias risk assessments (one for methodology and one for patient selection) and identified 48 with a “high risk of patient selection bias” and 36 with “high or unclear risk of bias from performance or interpretation of the serological test.” The authors also noted that only four of the forty studies including outpatients and only two studies evaluated point-of-care testing. The authors concluded that “currently, available evidence does not support the continued use of existing point-of-care serological tests” but acknowledged that “higher quality clinical studies assessing the diagnostic accuracy of serological tests for covid-19 are urgently needed” (Lisboa Bastos et al., 2020).
Kontou et al. (2020) performed a meta-analysis investigating the use of antibody tests in detecting SARS-CoV-2. The authors focused on IgG and IgM tests based on enzyme-linked immunosorbent assays (ELISA), chemiluminescence enzyme immunoassays (CLIA), fluorescence immunoassays (FIA), and lateral flow immunoassays (LFIA). A total of 38 studies encompassing 7848 individuals (3522 COVID-19 cases, 4326 healthy controls) were included. Of the 38 studies, 21 included data for both COVID-19 cases and controls. Fourteen studies using ELISA were included, and the authors found that IgG and IgM perform “similarly” individually, but in combination, resulted in a sensitivity of 0.935. Thirteen studies using CLIA resulted in an IgG sensitivity of 0.944, an IgM sensitivity of 0.810, and a combined IgG/IgM sensitivity of 0.910. The specificities ranged from 0.954 to 0.984. Thirteen studies used LFIA and found the IgG and IgM sensitivities to range from 0.53-0.66. Combining IgG and IgM resulted in sensitivities of 0.78-0.83. The authors also attempted to analyze FIA-based studies but were unable to due to the paucity of studies (three identified). The authors concluded that ELISA- and CLIA-based testing performed better sensitivity-wise and that LFIA studies are “more attractive for large seroprevalence studies but show lower sensitivity” (Kontou et al., 2020).
Ko et al. (2020) investigated the differences in neutralizing antibody production between asymptomatic and “mild” symptomatic COVID-19 patients, compared to pneumonic COVID-19 patients. A total of 70 patients (15 asymptomatic, 49 mild symptomatic, and six pneumonic) were included. A microneutralization assay was performed, along with a FIA and ELISA. Neutralizing antibody production was observed in all the pneumonic patients, 93.9% of the mildly symptomatic patients, and 80% of the asymptomatic patients. Further, the entire pneumonic group showed “high” titer (defined as ≥1:80), while 36.7% of the mild group and 20% of the asymptomatic group showed high titer. Both the FIA (for IgG) and ELISA detected anti SARS-CoV-2 at a high sensitivity (98.8% and 97.6% respectively). The authors concluded that “Most asymptomatic and mild COVID-19 patients produced the neutralizing antibody, although the titers were lower than pneumonia patients” (Ko et al., 2020).
Wu et al. (2020) investigated the association between levels of neutralizing antibodies (NAbs) and clinical characteristics in recovered COVID-19 patients. A total of 175 patients with “mild” symptoms of COVID-19 were included. The authors found that NAbs were detected in patients starting in days 4- 6 and reached peak levels in days 10-15. NAbs were also found not to cross-react with SARS-associated CoV,but correlated with “spike-binding antibodies targeting S1, receptor binding domain, and S2 regions. The authors also noted that NAbs titers were “significantly” higher in 56 “older” patients (1537 [IQR, 877-2427]) and 63 “middle-aged” patients (1291 [IRQ, 504-2126]) compared to 56 “younger patients” (459 [IQR, 225-998]). The authors concluded that “…NAb titers to SARS-CoV-2 appeared to vary substantially. Further research is needed to understand the clinical implications of differing NAb titers for protection against future infection” (Wu et al., 2020).
Kweon et al. (2020) collected 97 samples from patients with COVID-19 to analyze the serologic profiles and time kinetics of IgG and IgM against SARS-CoV-2 using the AFIAS COVID-19 Ab (BodiTechMed, 2024) and the EDI™ Novel Coronavirus COVID-19 ELISA Kit (EpitopeDiagnostics, 2024). The AFIAS assay uses recombinant nucleocapsid protein as an antigen to determine IgG and IgM antibodies against SARS-CoV-2 within 20 minutes from whole blood, serum, or plasma. The EDI™ ELISA Kit uses the microplate-based enzyme immunoassay technique to detect antibodies by measuring the optical densities (ODs) of each well of immunocomplexes. To determine the kinetics of antibodies, studies were performed at different past symptom onset (PSO) periods and to determine diagnostic accuracy of serologic assays, diagnostic sensitivity and specificities were calculated by PSO of ≤14 days and >14 days. Kinetic studies showed that “with both assays, IgM and IgG rapidly increased after seven days post symptom onset (PSO). IgM antibody levels reached a peak at 15–35 d PSO and gradually decreased. IgG levels gradually increased and remained at similar levels after 22–35 d” (Kweon et al., 2020). The diagnostic accuracy of both serologic assays also differed based on PSO. “The sensitivity of IgG samples from ≤14 d PSO was as low as 35.7%~57.1%, but it sharply increased for >14 d PSO to 88.2%~94.1%. This means that almost all patients with COVID-19 showed seroconversion after 14 d PSO, and IgG seronegative subjects in this period are considered less likely to be infected with SARS-CoV-2. In addition, both assays showed 94.2~96.4% of IgG specificities and increased IgG titers in COVID-19 patients were maintained. Thus, IgG serologic assays can be useful for ruling out SARS-CoV-2 infection after 14 d PSO, detecting past infection, and epidemiologic surveys” (Kweon et al., 2020). For IgM, the sensitivities were “as low as 21.4% (same in both assays) in the samples collected ≤14 d PSO and 41.2%~52.9% in samples >14 d PSO. These findings indicated that in patients infected with SARS-CoV-2, IgM seroconversion may not develop or might not be detected until the middle or late stages of infection. In other words, SARS-CoV-2 infection may be missed based on IgM seropositivity; thus, IgM tests must not be solely used in COVID-19 diagnosis and should be used only as a supportive tool in addition to molecular tests” (Kweon et al., 2020). In addition, IgM titers in COVID-19 patients showed a significant reduction after 35 d PSO; therefore, their utility in detecting past infection is limited. The author concludes that “testing for antibodies against SARS-CoV-2, especially IgG, has the potential for ruling out SARS-CoV-2 infection after 14 d PSO, detecting past infection, and epidemiologic surveys” (Kweon et al., 2020).
Caturegli et al. (2020) performed a case-control study to determine the clinical utility and validity of using SARS-CoV-2 antibodies, which were serum IgG and IgA antibodies formed against the SARS-CoV-2 spike protein detected by enzyme-linked immunosorbent assay (ELISA). When assays were formed 14 days or later after symptom onset, the researchers found that the sensitivity was 0.976 (95% CI, 0.928 to 0.995) and specificity was 0.988 (95% CI, 0.974 to 0.995), but the sensitivity decreased at earlier time points. Antibodies “predicted the odds of developing acute respiratory distress syndrome, which increased by 62% (CI, 48% to 81%; P < 0.001) for every 2-fold increase in IgG.” This demonstrates the linkage of antibodies used to measure clinical severity and for those who tested negative by NAAT but remained potentially COVID-positive.
In a household cohort study, Churiwal et al. (2021) assessed the utility of a rapid point of care test for COVID-19 antibodies by comparing the performance of BioMedomics COVID-19 IgM/IgG Rapid Antibody Test against an ELISA. The test was performed on 303 patients at study enrollment and four weeks later. According to the results, sensitivity was lower early in infection and those who never developed symptoms (74% sensitivity). Only two were detected among 499 tests early in infection due to false-positive IgM bands. When measured four weeks later after the onset of symptoms, it demonstrated robust sensitivity (90%) and complete specificity (100%). The authors conclude that "When used appropriately, rapid antibody tests offer a convenient way to detect symptomatic infections during convalescence” (Churiwal et al., 2021).
Fox et al. (2022) performed a meta-analysis to assess the accuracy of antibody tests. The analysis covered 178 studies with a total of 64,688 samples taken from 25,724 people with confirmed SARS-CoV-2. All the studies were conducted before the introduction of the SARS-CoV-2 vaccines to ensure the responses were due to naturally acquired antibodies. The average sensitivity for either IgG or IgG combined with IgM was 41.1% one week after symptom onset, 74.9% two weeks after symptom onset, and 88.0% three weeks after symptom onset. The average sensitivity during the convalescent phase of infection, up to 100 days since symptom onset, was 89.8% for IgG, 92.9% for IgG or IgM combined, and 94.3% for total antibodies. The average sensitivities for IgM alone “followed a similar pattern but were of a lower test accuracy in every time slot.” The authors conclude that antibody tests “could be a useful diagnostic tool” but note that “antibody tests have an increasing likelihood of detecting an immune response to infection as time since onset of infection progresses and have demonstrated adequate performance for detection of prior infection for sero-epidemiological purposes” and “the applicability of results for detection of vaccination-induced antibodies is uncertain” (Fox et al., 2022).
Antigen Testing
Another possible diagnostic testing methodology is antigen detection testing, which relies upon the direct detection of parts of the virus called “antigens”—in this instance, proteins located on the outside of SARS-CoV-2, such as the spike protein (S) or nucleocapsid protein, that can cause an immune response in an individual. What makes this method of testing distinct from antibody testing is that antigen testing directly measures the presence of the virus in a person whereas antibody testing is measuring the patient’s response to an infection. These antigen detection tests can be deployed as rapid antigen tests that decrease the turnaround time for results but usually lack specificity (Loeffelholz & Tang, 2020).
On May 8, 2020, the FDA issued the first EUA for antigen testing for COVID-19 to the Quidel Corporation for their Sofia®2 SARS Antigen FIA lateral flow immunofluorescent sandwich assay for the qualitative detection of the nucleocapsid (N) protein antigen of SARS-CoV-2 for use in individuals suspected of COVID-19 by their healthcare provider (Quidel Corporation, 2020). This test has been approved as a point-of-care (POC) test (FDA, 2024c). This test functions by detecting the N protein of either the SARS-CoV or SARS-CoV-2 virus from an upper respiratory sample (either a nasal swab or nasopharyngeal swab). First, the sample is placed in a reagent tube so that any virus, if present, is broken apart to allow for the N proteins to be exposed. The sample then travels from the sample well, down a test strip—where the term “lateral flow” is derived—where the proprietary reagents will recognize any N proteins and trap them in place on the strip. The test requires at least 15 minutes to develop prior to analysis. The strip can then be read by the Sofia®2 system that measures the fluorescent signal from the proprietary reagents. The Sofia®2 system allows the user to have two different modes for analysis—“Walk Away” and “Read Now.” For the “Walk Away” mode, the user will insert the test cassette strip into the system, and the results will be displayed in 15 minutes because the test will be developed while in the instrument. In “Read Now” mode, the user must have already allowed at least 15 minutes for the test to develop prior to inserting it into the instrument. Then, the Sofia®2 system will display the result within one minute (Quidel Corporation, 2020). On August 20, 2020, Quidel reported that the Sofia test’s labeling had been amended to include “either nasal or nasopharyngeal swabs” thereby allowing Quidel a second corresponding kit configuration (BioSpace, 2020).
On July 2, 2020, a second antigen test (BD Veritor System for Rapid Detection of SARS-CoV-2) from Becton, Dickinson, and Company was issued an EUA. This test is described as “a chromatographic digital immunoassay intended for the direct and qualitative detection of SARS-CoV-2 nucleocapsid antigens in nasal swabs from individuals who are suspected of COVID-19 by their healthcare provider within the first five days of the onset of symptoms.” The test is authorized for use in POC settings. The test’s mechanism of action is as follows: if there are any antigens in the sample (in this case, the nucleocapsid of the virus), they will bind to antibodies conjugated to detector particles in the test strip. The new “conjugates” migrate to the “reaction area” and are captured by another line of antibodies. The test reads positive when the conjugate is found at both “Control” and “Test” positions on the device. BD Veritor reported the following values for the test (in comparison to RT-PCR): 84% positive predictive agreement, 100% negative predictive agreement, 98% overall percent agreement, 100% positive predictive value, and 97.5% negative predictive value. No cross-reactivity was reported (BD Veritor, 2020).
On August 18, 2020, a third antigen test (LumiraDx SARS-CoV-2 Ag Test from LumiraDx UK Ltd.) was issued an EUA. The test is described as “a single use fluorescence immunoassay device designed to detect the presence of the nucleocapsid protein antigen directly from SARS-CoV-2 in nasal swab specimens, without transport media.” The mechanism of action is as follows: when a droplet of the specimen is added to the “Test Strip”, pre-made reagents on the strip react with any antigen in the specimen. The amount of fluorescence created is proportional to the amount of antigen detected. LumiraDx reported a limit of detection of 32 TCID50/mL [tissue culture infectious dose], as well as a 97.6% positive percent agreement, 96.6% negative percent agreement, 93.1% positive predictive value, 98.8% negative predictive value, and 96.9% overall percent agreement (based on 257 total samples) (LumiraDx, 2020).
As of April 20, 2022, 50 antigen tests have Emergency Use Authorization (EUA) by the U.S. Food and Drug Administration (FDA) (FDA, 2023a) These testing methods include (among others): Bulk Acoustic Wave (BAW) Biosensors, Chemiluminescence Immunoassays, Chromatographic Digital Immunoassays, Digital Lateral Flow, Magnetic Force-assisted Electrochemical Sandwich Immunoassay (MESIA), Microfluidic Immunofluorescence Assay, and Paramagnetic Microbead-based Immunoassay (FDA, 2023a).
Clinical Utility and Validity of Antigen Testing
To address the clinical performance, two primary studies were performed. Both studies only used frozen samples. The first study used 143 samples with 80% PPA or Positive Percent Agreement (47/59 of positive samples tested “positive”). They report 100% NPA or Negative Percent Agreement—all 84 negative samples tested “negative.” The second study used a total of 48 samples. Again, 80% of the positive samples tested “positive”; however, only a total of five positive samples were included within this second study. The remaining 43 samples were all negative samples. This study reports a sensitivity of 80.0%, but a 95% confidence interval range of 37.6% - 96.4%. A third supportive study was also performed. In this study, thirty swabs were taken. Twenty of these swabs were spiked with one lower concentration of the virus while the remaining ten swabs were spiked with a higher concentration of the virus. Then, all 30 swabs were tested and compared to 47 control (“unspiked”) samples. In this study, none of the “unspiked” control samples tested “positive” while all 30 of the “spiked” samples, regardless of the concentration, tested positive. Quidel also tested the LoD of the Sofia®2 SARS Antigen FIA test. LoD is typically measured by determining the TCID50 (median tissue culture infective dose). The TCID50 is the amount where 50% of the cells within a sample are infected.(Wulff et al., 2012). For the Sofia®2 SARS Antigen FIA test, the LoD for a direct swab sample has a TCID50 of 113 mL whereas it is 850 mL if the initial sample is from a swab sample that has been diluted into three mL of reagent. Finally, Quidel also checked this antigen test for possible cross-reactivity with several microorganisms and other viruses. It shows no cross-reactivity with any of the microorganisms or viruses tests other than SARS-CoV. Of note, it does not cross-react with human coronavirus 229e, OC43, NL63, or MERS-CoV (heat-inactivated); however, they did not check for possible cross-reactivity with the other known human coronavirus (HKU1) due to a lack of availability at this time. This is noteworthy since this coronavirus is associated with the common cold. Limitations of the Sofia®2 SARS Antigen FIA test includes the following:
- This test must be performed using the Sofia®2 system, and the test must be performed accurately following the test procedure. Failure to do so can adversely affect the performance of the test and may invalidate the results.
- A positive test cannot distinguish between a SARS-CoV or a SARS-CoV-2 infection. SARS-CoV is the virus that caused the SARS outbreak of 2003. It should be noted that there is no current outbreak of SARS.
- This test also does not distinguish between “live” (viable) virus and non-viable virus. Consequently, the test results do not necessarily correlate with viral culture results performed on the same sample.
- This test is only for the qualitative use on a sample from either a nasal swab or a nasopharyngeal swab. It has not been approved for use, at this time, on any other sample, such as saliva.
- Negative test results can occur if the viral level is below the lower limit of the test. All negative results “should be treated as presumptive and confirmed with an FDA authorized molecular assay, if necessary, for clinical management, including infection control” (Quidel Corporation, 2020)
- Positive test results do not rule out coinfections, and negative results do not “rule in” other non-SARS viral or bacterial infections.
- The clinical performance assays submitted for FDA approval were performed using frozen samples; the test may have a different performance when used with a fresh sample (such as in a point-of-care setting).
- “If the differentiation of specific SARS viruses and strains is needed, additional testing, in consultation with state or local public health departments is required (Quidel Corporation, 2020).”
- As previously noted, the company did not check this test (as of publication date) for cross-reactivity with human coronavirus HKU1 due to a lack of availability of that strain. This is notable since this virus is associated with upper respiratory conditions such as the common cold.
One multi-center study, currently a preprint at the time of publication, reports the development of another rapid antigen detection test (RADT) that screens for SARS-CoV-2 by targeting the nucleocapsid protein. This test, when using a nasopharyngeal swab sample, reports a 100% positive agreement with RT-PCR testing. They also report 73.6% positive agreement when using a urine sample (Diao et al., 2020). This study is yet to be published in a peer-reviewed journal, and the test is not FDA approved as of May 18, 2020. Another study published recently in ACS Nano reports on the development of a RADT using field-effect transistor (FET)-based biosensing where a graphene sheet for the FET is coated with a specific antibody against the SARS-CoV-2 spike protein. This method can detect the protein in concentrations as low as one fg/mL in buffer and has an LOD of 242 copies/mL for a clinical sample (versus 16/mL for a culture medium) (Seo et al., 2020). To date, the WHO states that “Ag-RDTs could play a significant role in guiding patient management, public health decision making and in surveillance of COVID-19. Currently, there is insufficient evidence on performance and operational use to recommend specific commercial products” (WHO, 2021a).
Scohy et al. (2020) evaluated the Coris COVID-19 Ag [Antigen] Respi-Strip test in comparison to RT-PCR. The authors tested 148 nasopharyngeal swabs, with 106 testing positive by RT-PCR. The rapid antigen test detected 32 of these 106 positive results, for a sensitivity of 30.2%. All samples deemed positive by the antigen test were also deemed positive by RT-PCR. The authors noted that higher viral loads were associated with better detection by antigen tests but concluded that “the overall poor sensitivity of the COVID-19 Ag Respi-Strip does not allow using it alone as the frontline testing for COVID-19 diagnosis” (Scohy et al., 2020).
Mak et al. (2020) evaluated the BIOCREDIT COVID-19 Ag test in comparison to RT-PCR. The BIOCREDIT test’s limit of detection (LOD) was compared to RT-PCR and viral culture, and a total of 368 samples from confirmed COVID-19 cases were included. A sample volume of 100 μL was used. The authors found the LOD of BIOCREDIT to be 1000-fold less sensitive than viral culture (BIOCREDIT LOD: 10-2 , viral culture: 10-5 ). RT-PCR’s LOD was measured to be 10-7 . Further, BIOCREDIT detected between 11.1% and 45.7% of RT-PCR positive patients from COVID-19 patients. The authors concluded that “This study demonstrated that the RAD test serves only as adjunct to RT-PCR test because of potential for false-negative results” (Mak et al., 2020).
Lambert-Niclot et al. (2020) analyzed the COVID-19 Ag Respi-Strip test and compared its accuracy to RT-PCR. A total of 138 nasopharyngeal samples were included, with 94 testing positive by RT-PCR.
The Respi-Strip test identified 47 of 94 positive specimens for a sensitivity of 50%, although the specificity was 100% for both tests. The authors also noted that the control lines were “barely” visible for 17 tests (nine positive and eight negative). The authors acknowledged that due to the low prevalence in France (the country in which this study was performed), prospective studies should be undertaken(Lambert-Niclot et al., 2020).
Hirotsu et al. (2020) evaluated a new antigen test (LUMIPULSE) which is based on chemiluminescence enzyme immunoassay. A total of 313 nasopharyngeal swabs were included (82 serial samples from seven COVID patients, 231 individual samples from four COVID patients and 215 healthy controls). These samples were tested by both LUMIPULSE and RT-PCR. Compared to RTPCR, LUMIPULSE demonstrated a 91.4% overall agreement rate (286/313), with a 55.2% sensitivity and 99.6% specificity. At >100 viral copies, LUMIPULSE agreed perfectly with RT-PCR, and at 10- 100 viral copies, there was an 85% concordance rate (with concordance declining at lower viral loads). The authors concluded that “the LUMIPULSE antigen test can rapidly identify SARS-CoV-2-infected individuals with moderate to high viral loads and may be helpful for monitoring viral clearance in hospitalized patients” (Hirotsu et al., 2020).
Villaverde et al. (2021) conducted a multicenter study to compare the diagnostic accuracy of the Panbio coronavirus disease 2019 Antigen Rapid Test of nasopharyngeal samples in pediatric patients with COVID-19 symptoms ≤5 days. They demonstrated “limited accuracy in nasopharyngeal antigen testing: overall sensitivity was 45.4%, and 99.8% of specificity, positive-predictive value was 92.5%,” with moderate concordance between the RT-PCR and antigen test. They noted that a high proportion of false-negative results from the antigen tests (54.5%) may have public health implications in unknown spreading of the virus. But because this test has a good positive likelihood ratio, and is cheap, rapid, and widely distributed, it may be used as a first screening test in a pandemic situation, though its value as a diagnostic tool is questionable due to the low sensitivity and negative likelihood ratio.
Peacock et al. (2022) studied the clinical utility of the BinaxNOW antigen test by Abbott Diagnostics, a lateral flow immunochromatographic point-of-care test which provides results in 15 minutes from a nasal swab. BinaxNOW was performed on 735 samples and results were compared to PCR. In total, 623 of 735 (84.8%) had symptoms and 460 of 623 patients (62.6%) had symptoms for less than seven days. Positive tests occurred in 173 (23.5%) for the PCR and 141 (19.2%) with the BinaxNOW test. Those with symptoms for more than two weeks had a positive test rate half of those with earlier onset. " In patients with symptoms ≤7 days, the sensitivity, specificity, and negative and positive predictive values for the BinaxNOW test were 84.6%, 98.5%, 94.9%, and 95.2%, respectively" (Peacock et al., 2022). The authors conclude that BinaxNOW has good sensitivity and specificity and is recommended for patients with symptoms up to two weeks (Peacock et al., 2022).
Panel Testing
Multiple laboratories have developed panels to screen for possible microorganism infections from a single sample. For example, multiplex PCR can simultaneously detect multiple pathogens rather than sequentially testing for each individual pathogen. Such testing can be advantageous when different pathogens may manifest with similar clinical presentation; however, this testing can be costly and can also result in false-negatives if preferential amplification of one target over another occurs. As of May 4, 2022, the BioFire® Respiratory Panel 2.1 (RP2.1), the QIAstat-Dx® Respiratory SARS-CoV-2 Panel, ePlex Respiratory Pathogen Panel 2, cobas SARS-CoV-2 & Influenza A/B, Xpert Xpress SARS-CoV-2/Flu/RSV, Quest Diagnostics RC COVID-19 +Flu RT-PCR, Sofia 2 Flu + SARS Antigen FIA, and the Influenza SARS-CoV-2 (Flu SC2) Multiplex Assay from the CDC received an EUA from the FDA for testing for COVID-19 (FDA, 2024c). The BioFire® Respiratory Panel 2.1, the QIAstat-Dx® Respiratory SARS-CoV-2 Panel, and ePlex Respiratory Pathogen Panel 2 use multiplex nucleic acid testing from a nasopharyngeal swab to detect and differentiate microorganisms listed in Table 1 (BioFire, 2020; GenMark Diagnostics, 2024; Qiagen GmbH, 2021), whereas the CDC Multiplex detects and differentiates influenzas A and B from SARS-CoV-2 (FDA, 2021c).